Introduction
As gas consumption demands increase, hydrogen generators are now an essential part of many laboratories. The generator provides high-purity gas on demand in a very short period of time, which is superior to gas cylinders, especially in terms of health and safety, because storing high-pressure hydrogen in a laboratory working environment can cause concern. .
The generator typically contains less than half a liter of hydrogen, which is almost negligible compared to 9,000 liters of gas in a 50-liter pressure cylinder. A number of hydrogen generator manufacturers produce high-quality instruments that provide ultra-high purity gases for a variety of applications, including nanotube research, gas chromatography, and the semiconductor industry. Many people know that hydrogen comes from the electrolysis of water, but the actual operation is really so simple? Why is the generator so expensive?
Although the use of gas generators to generate high purity hydrogen involves many highly confidential secrets, the generation of hydrogen from water can only utilize several basic mechanisms. Hydrogen generator systems typically use a two-phase system to produce purified hydrogen. Hydrogen is first separated from the water and then purified. Many hydrogen generators use a proton exchange membrane (PEM) with a hydrogen purification system such as a palladium diffusion or pressure swing adsorption (PSA) dryer.
The proton exchange membrane for hydrogen generation works as efficiently as a reverse operated fuel cell. Water is electrolyzed at the PEM, which is a solid polymer electrolyte that promotes the movement of H+ ions, while O2- ions are fixed and form O2 molecules (see Figure 1.). Hydrogen ions are transported along the ion channel by the PEM lattice by means of surface diffusion, Grottuss diffusion, and carrier diffusion. Proton transfer is accomplished by transmembrane proton transfer, which is capable of penetrating cations but is impermeable to anions or electrons and can only deliver hydronium ions. 2 The hydrogen collected by the cathode of the electrolytic cell needs to be purified. Purification can be done in a variety of ways.
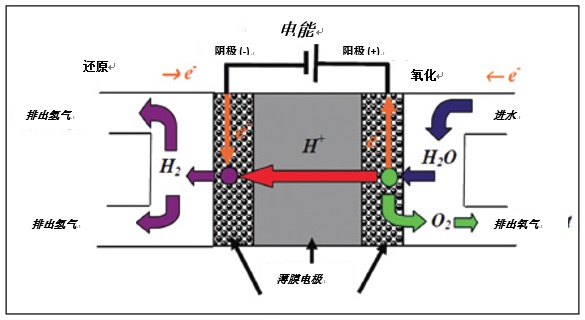
Figure 1. Functional schematic of the proton exchange membrane
Palladium electrolyzer / purification integrated system
In a palladium electrolyzer, hydrogen separation/purification system, a palladium anode consisting of a bundle of tubes and a palladium cathode (see Figure 2) are used to electrolyze water containing a soluble electrolyte, typically NaOH or KOH. As the electrolysis current passes through the solution, hydrogen ions diffuse through the cathode of the palladium tube, producing ultra-high purity hydrogen.

Figure 2. Schematic of a hydrogen generator using an integrated system for electrolysis and purification
The system provides ultra high purity hydrogen and minimal moisture with O2 carrying. However, the electrolyte in the battery must be replaced periodically, which means that the generator requires at least 12 hours of downtime (including cooling and start-up time). When replacing the electrolyte, all solutions need to be replaced. The remaining sulfur compounds and unsaturated hydrocarbons reduce the permeability of the palladium tubes to hydrogen ions, so the palladium tubes also need to be replaced periodically.
Palladium diffusion membrane
The palladium diffusion membrane drives H+ ions through the palladium membrane by pressure. Palladium/silver alloy films can selectively diffuse hydrogen ions through the film at temperatures above 300, while allowing impurities such as H2O, CO2 or CO to pass through and remain inside the film. These impurities can be discharged to the air later. Palladium diffusers come in a variety of styles, including tube arrays, spiral tubes or film foils. After the hydrogen ions pass through the membrane, pressurized diatomic hydrogen molecules are formed and transported to the analytical instrument (see Figure 3.).
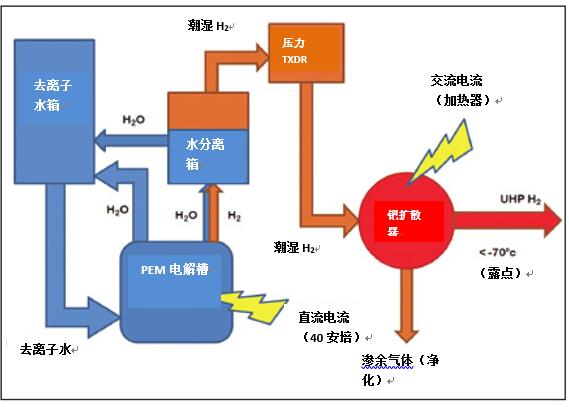
Figure 3. Schematic diagram of a hydrogen generator for hydrogen purification using a palladium diffusion membrane
However, despite the high purity of hydrogen, the life of the palladium cell is relatively short (3 to 5 years), and the presence of dissolved hydrogen in the film during water supply or power failure causes embrittlement to form flow or impurity changes, resulting in mechanical stress. It will easily cause the palladium battery to malfunction.
PEM/PSA
To purify and dry the hydrogen, pressure swing adsorption works by changing the flow of hydrogen through two parallel columns of zeolite adsorbent material, which acts as a molecular sieve (see Figure 4.), allowing smaller hydrogen molecules to pass through while Keep larger water molecules. Hydrogen is purified as it passes through the A-column, and a small amount of dry gas is discharged backward through the B-pillar to purify the retained moisture by means of the adsorbent material. When the A column reaches the optimum adsorption, the process is reversed. At this time, the B column takes over the A column for hydrogen purification and the A column for regeneration. The two columns are converted between hydrogen purification and regeneration, allowing the system to continuously produce purified hydrogen with negligible pressure fluctuations and pulsation effects. After each cycle is completed, the system is fully regenerated, so there is no need to change materials.
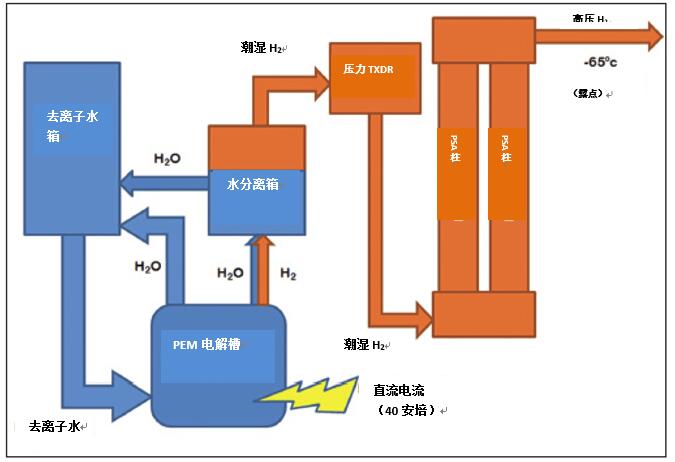
Figure 4. Schematic diagram of a hydrogen generator for hydrogen purification using pressure swing adsorption
Another optional drying system for PSA that can be used with PEM is the silica gel drying system (see Figure 5.). The system only removes water from the PEM-derived hydrogen through the silica gel column. The system is cheaper than PSA and palladium drying systems, but the high purity hydrogen produced contains more water and oxygen. The system has a relatively low maintenance cost, and it is only necessary to periodically replace the deionizer box and the drying column as needed.
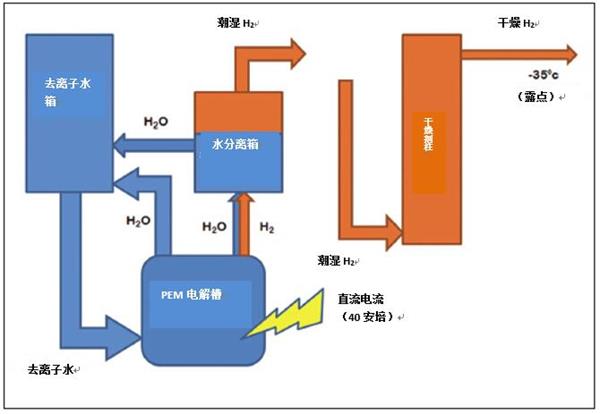
Figure 5. Schematic diagram of a hydrogen generator using a silica gel column for hydrogen purification.
Advantages and disadvantages
Studies have shown that a palladium purification system can produce the driest hydrogen 1 , but there are many advantages and disadvantages to using the system. By using the selective permeability to H+, it is easy to cause palladium embrittlement when the H2 is present in the film under low temperature conditions. Palladium cells operate at high temperatures, so complex startup and shutdown procedures must be performed to avoid embrittlement and ultimately damage to the cell. The replacement cost of the palladium electrolytic cell is relatively high and its life is relatively short.
On the other hand, PSA has advantages over palladium systems in terms of robustness and lower maintenance requirements. PSA does not need to operate at high temperatures and high electrolysis currents, and maintenance costs are low. Although PEM/PSA can achieve a purity slightly lower than that of a palladium system (mass 6.0 and 7.0), the PSA system requires no shutdown and the system achieves high purity in 2 to 3 hours.
All four of these systems produce high purity hydrogen, eliminating the need to store hydrogen bottles in the work environment. The cost of the generator is usually reflected in the quality of the gas produced and the technology used to produce the gas. Your choice of generator usually depends on the application of hydrogen and the gas purity requirements of the application.
three-way y-type gas valve,angiography hemostasis valve set,hemostasis valve set,Y-Valve
Anesthesia Medical Co., Ltd. , https://www.trustfulmedical.com