Hemorrhagic disease RNAi therapy restart clinical trial
December 18, 2017 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Sanofi Genzyme and Alnylam announced today that the US FDA approved the restart of fitusiran's clinical research on hemophilia, including the Phase 2 Open Label Extension Study and the Phase 3 Research Program ATLAS.

Hemophilia is an inherited bleeding disorder. The patient does not produce enough thrombin to ensure effective blood clotting, resulting in repeated bleeding of muscles and major organs. About 400,000 people worldwide suffer from hemophilia A or B. Current standard treatments for hemophilia patients include prophylactic or "on-demand" clotting factor replacement that temporarily restores blood clotting capacity. However, for current replacement therapy, hemophiliacs have a risk of developing neutralizing antibodies or replacement factor "inhibitors" that affect about one-third of patients with hemophilia A and some patients with hemophilia B, For patients who developed a replacement factor inhibitor, their chances of hospitalization due to bleeding time doubled.
Fitusiran is an RNAi therapy that targets anti-thrombin, administered subcutaneously once a month for the treatment of hemophilia A and B. This method aims to reduce the level of antithrombin so that there is enough thrombin in the body to stop bleeding and prevent bleeding. Fitusiran also has the potential to be used in rare bleeding disorders.
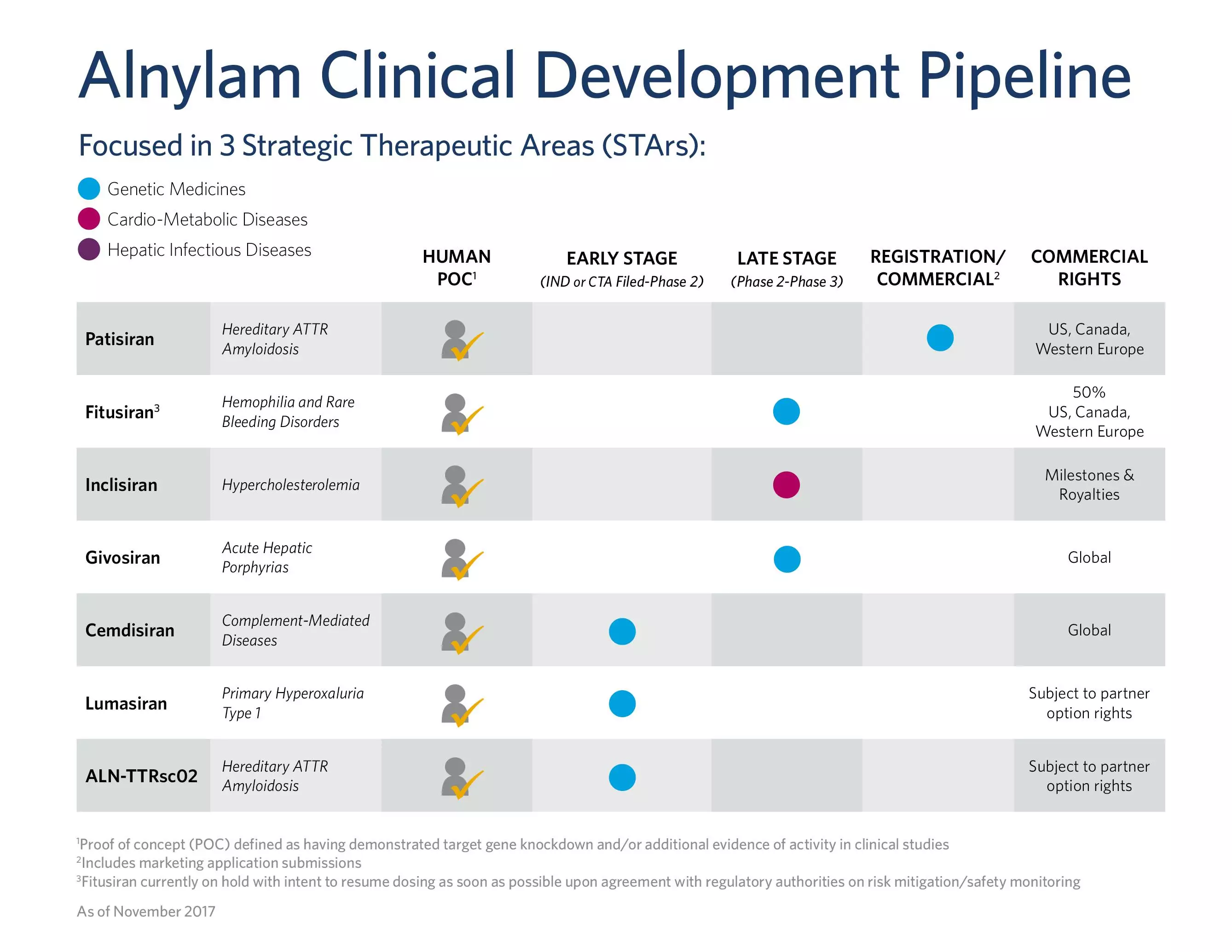
â–²Fitusiran is one of Alnylam's main products (Source:Fitusiran official website)
Four months ago, in a clinical study, a patient with hemophilia A died after receiving treatment with fitusiran, after which the FDA suspended Alnylam's clinical trial of flusymic disease with fitusiran. Alnylam and the FDA have recently agreed on new clinical risk mitigation measures. The FDA approved protocol revisions and other updated clinical data for the fitusiran study.
Dr. Akin Akinc, vice president and general manager of Altylam's fitusiran division, said: "We are pleased that the FDA has decided to restart clinical trials because fitusiran may help improve the lives of people with hemophilia. With additional risk mitigation measures in place, we look forward to The later development of fitusiran is expected to resume use before the end of the year."
We are concerned about the progress of this new drug, and we look forward to the early treatment of patients with hemophilia.
Reference materials:
[1] Finally Some Good News as the FDA Lifts Hold on Sanofi, Alnylam's Fitusiran
[2] Alnylam's official website
[3] Sanofi Genzyme Official Website
Original title: Green light release! Hemorrhagic disease RNAi therapy restart clinical trial
Water Purifier,Water Purifier System,Household Water Purifier,Commercial Water Purifiers
Jinan Mucho Commercial Inc. , https://www.muchovet.com